Event JSON
{
"id": "cab24dec881d0cb561c5659fea5073caeb082670c3bd580d2b0412fc1beedae8",
"pubkey": "e9d555fcc508b077f9adb908fb97770593291651d3c4ae96d8d9a04d2312ba7a",
"created_at": 1725046390,
"kind": 1,
"tags": [
[
"d",
"deacbafe-e0cf-43ab-8612-255c7ea07fe1"
],
[
"subject",
"Summary of FDA authorizes Novavax’s updated Covid vaccine, paving way for fall rollout"
],
[
"r",
"https://layer3.news/api/public/publications/deacbafe-e0cf-43ab-8612-255c7ea07fe1"
],
[
"snaid",
"6edd3227-e00c-4e68-bc80-50c832580e7f"
],
[
"p",
"e9d555fcc508b077f9adb908fb97770593291651d3c4ae96d8d9a04d2312ba7a"
],
[
"p",
"b84f613c130f4cd316c9593bf9024eb6278ec137af90eff28033712bdda04ced"
],
[
"q",
"e60b44633475776ecc5ba8788b36806aac39e5d6ec6d694926729efe86fd5a4f",
"wss://articles.layer3.news"
],
[
"e",
"e60b44633475776ecc5ba8788b36806aac39e5d6ec6d694926729efe86fd5a4f",
"wss://articles.layer3.news",
"root"
]
],
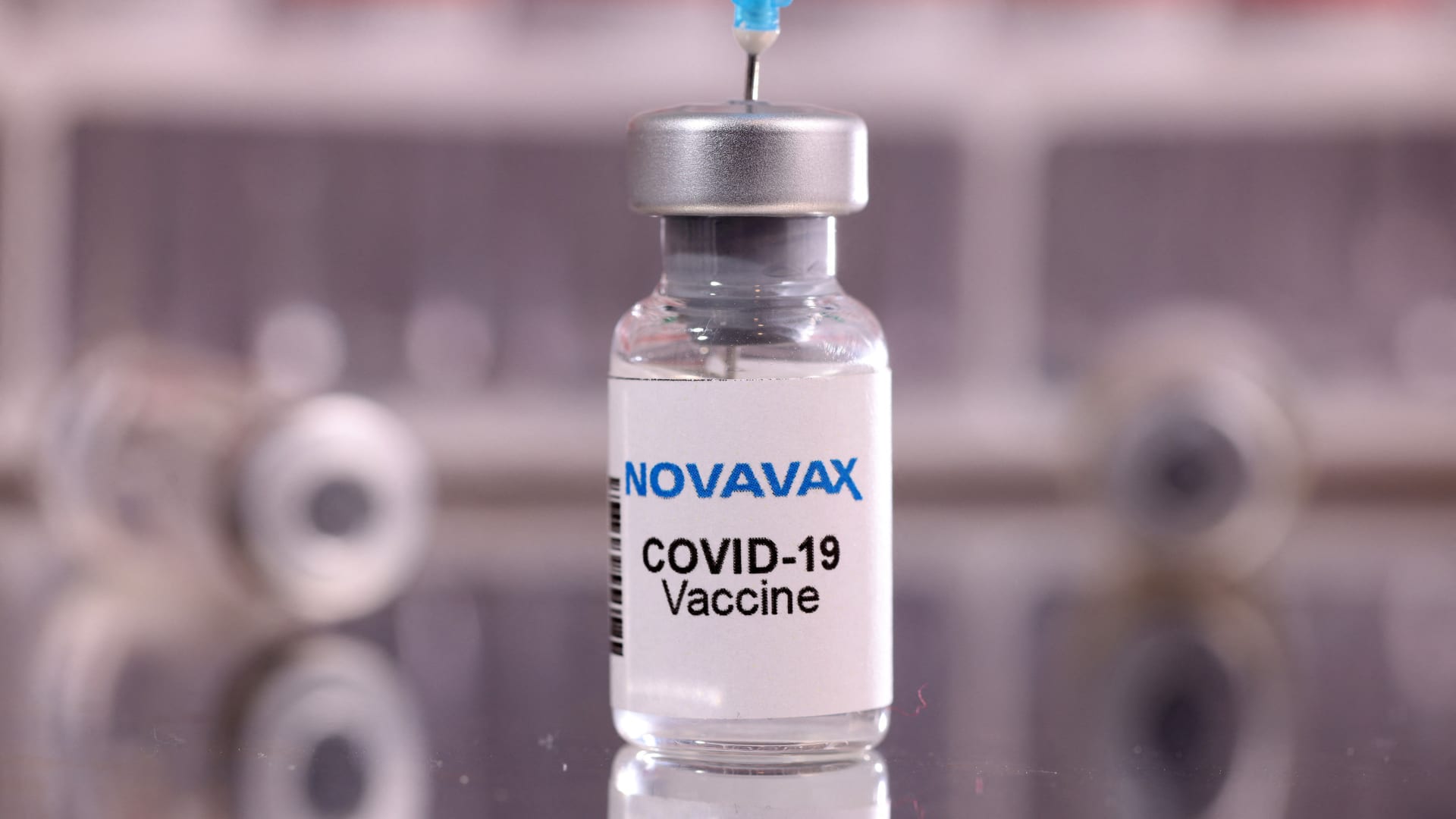
"content": "Summary of FDA authorizes Novavax’s updated Covid vaccine, paving way for fall rollout\nnostr:nevent1qqswvz6yvv682amwe3d6s7ytx6qx4tpeuhtwcmtffyn898h7sm745nczyzuy7cfuzv85e5cke9vnh7gzf6mz0rkpx7hepmljsqehz27a5pxw6qcyqqqqqqgtpyzdm\n\nThe FDA authorized Novavax's updated protein-based Covid vaccine for emergency use in people ages 12 and up, which targets the highly contagious omicron subvariant JN.1. The vaccine provides protection against descendants of JN.1 that are currently dominant in the U.S. and is a valuable alternative for people who don't want to take mRNA shots from Pfizer and Moderna.",
"sig": "60dd06e2cef51b29e26d7e0b784148dc9c98e625e774c62df49edd0bb2580797775015a3fa2a1d545cadbefe1ddf0ff49e1be01e234c01aa1812fef187606892"
}